Hot Melt Extrusion (HME) is a process of applying heat and pressure to melt a polymer and force it through a die in a continuous process. HME has been widely applied in the plastics industry, and more recently has been used to prepare several types of dosage forms and drug delivery systems for pharmaceuticals. The HME process is used to mix the active pharmaceutical ingredient (API) with a polymer and other excipients at elevated temperature and pressure. The extruded material can then be cut or milled into micro pellets to fill capsules or compress into tablets.

Application Requirements
A key component of the pharmaceutical extrusion process is its injection of CO2 directly into the extruder barrel, to expand the cell structure known as foaming, of the polymer/API. It also creates a porous 3D cell structure which increases the surface area of the polymer, resulting in higher dissolution rates or faster absorption of the API in the body. The CO2 injection allows the extruder to run at lower temperatures (-30C or lower) which prevents degradation of sensitive APIs. CO2 is also environmentally friendly, reliable, non-flammable, and low-cost. However, CO2, can be a difficult fluid to measure because it can change state – liquid to gas to supercritical – with even a slight change in pressure or temperature.
A piston pump was initially used to deliver the CO2, into the body of the extruder, but the pump did not provide verification that the CO2 was being properly delivered. The extruder manufacturer could only see that the pump was running at a specific speed – not the specific mass flow rate of the CO2 being delivered. The manufacturer needed a more accurate method of monitoring the CO2, they were injecting directly into the extruder barrel, to ensure the desired cell structure of the extruded material.
- Measure and control the mass flow rate of supercritical CO2
- Accurate flow rate at low temperatures (-30C or lower)
Process Solution
Highly reliable and accurate, the Brooks Instrument Quantim Coriolis mass flow meter controller was selected to measure and control the mass flow of the liquid CO2 injection, rather than simply controlling the pump speed. When CO2, is held at or above its critical temperature and pressure, as in the HME process, it is supercritical.
When a fluid is supercritical, it adopts properties midway between a gas and a liquid. At or near a critical point, fluid properties such as specific heat, density and viscosity vary significantly with small changes in temperature and pressure. This makes it difficult for most flow measurement technologies to provide accurate and repeatable technology is ideally suited for this application because the device measures true mass flow independent of fluid properties.
Introducing the Coriolis mass flow meter controller on the outlet side of the pump allowed the customer to monitor the precise mass flow of CO2 into the extruder and ensure it remained in its supercritical state by monitoring the fluid density. Integrating the Quantim meter controller output signal into the process control loop provided feedback to control the pump speed precisely based on the mass flow rate of the CO2 injection.
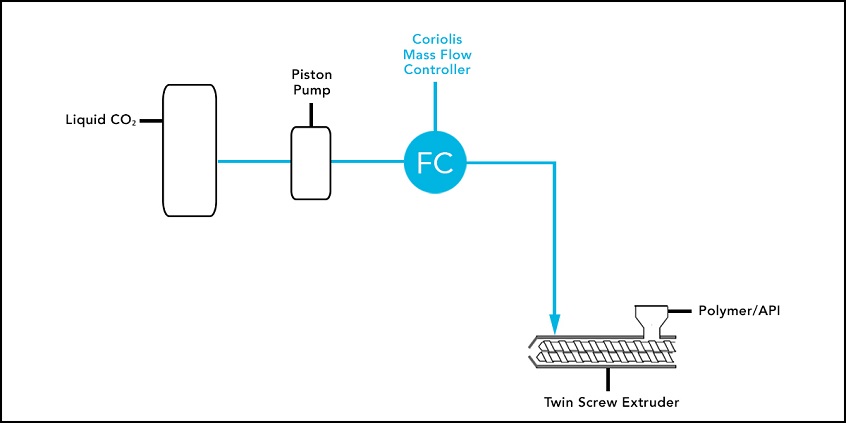
Flow scheme
The result was precise addition of the supercritical CO2 into the hot melt extrusion process, ensuring the desired cell structure of the extruded material and ultimately, its transformation into a potentially life-saving drug delivery system.

Component datasource missing. Select a datasource for this component.